Indica Labs Receives First FDA Clearance for HALO AP Dx Digital Pathology Platform

Indica Labs Receives First FDA Clearance for HALO AP Dx Digital Pathology Platform – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »Tariff Hikes Hit Medical Devices: Can Digital Manufacturing Provide Relief?

Manufacturers are grappling with the potential for disrupted supply chains and increased production costs as the US-China trade war intensifies. But companies can gain a competitive advantage by embracing digital manufacturing solutions.
Read the full article at: www.mddionline.com
Continue Reading »FDA clears Galvanize’s pulsed field needle for cancer, soft tissue ablation

The INUMI Flex endoscopic needle ablation system was cleared for use against soft-tissue lesions such as lung nodules that can harbor tumor cells.
Read the full article at: www.fiercebiotech.com
Continue Reading »Boston Scientific’s modular, wireless heart rhythm implant approach clears clinical study

A 6-month trial showed Boston Scientific’s pair of modular implants for managing cardiac rhythms—and correcting dangerous ones—could be successful.
Read the full article at: www.fiercebiotech.com
Continue Reading »New Medical Device Packaging Course a Hit

In the last two years, about 100 “students” have learned the Fundamentals of Medical Device Packaging and are putting the knowledge to good use.
Read the full article at: www.packagingdigest.com
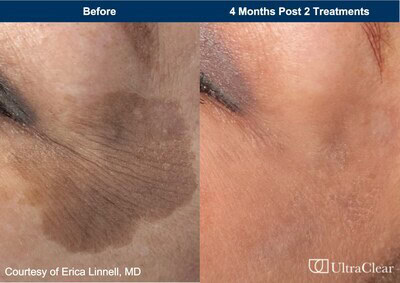
Continue Reading »Acclaro Medical Announces FDA Clearance of UltraClear® Cold Ablative Fractional 2910 nm Fiber Laser for Treatment of Benign Pigmented Lesions and Vascular Dyschromia

Acclaro Medical Announces FDA Clearance of UltraClear® Cold Ablative Fractional 2910 nm Fiber Laser for Treatment of Benign Pigmented Lesions and Vascular Dyschromia – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »5 Phases of FDA’s Final Rule on LDTs

Looking at the five stages of FDA’s phaseout policy concerning laboratory-developed tests.
Read the full article at: www.mddionline.com
Continue Reading »Karius snares FDA breakthrough tag for genomic infectious disease blood test

After picking up $100 million in venture capital cash earlier this month, Karius is adding another feather to its cap.
Read the full article at: www.fiercebiotech.com
Continue Reading »Teal Health Completes Clinical Trial at Record Speed and Receives FDA Breakthrough Designation for Its At-Home Cervical Cancer Screening Device, the Teal Wand™

Teal Health Completes Clinical Trial at Record Speed and Receives FDA Breakthrough Designation for Its At-Home Cervical Cancer Screening Device, the Teal Wand™ – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »FDA clears Masimo’s over-the-counter Stork baby monitoring system

Masimo secured FDA clearance for its Stork baby monitoring system that can help track certain vital signs in healthy infants up to 18 months old and provide alarms to parents or caregiver…
Read the full article at: www.fiercebiotech.com
Continue Reading »