New Study Highlights Benefits of Medication Management Automation in Long-Term Care Facilities

New Study Highlights Benefits of Medication Management Automation in Long-Term Care Facilities – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »Researchers develop handheld device for rapid bacterial detection
Hear the words E. coli or salmonella and food poisoning comes to mind. Rapid detection of such bacteria is crucial in preventing outbreaks of foodborne illness.
Read the full article at: www.news-medical.net
Continue Reading »Intuitive launches latest da Vinci robot, with force feedback controls

Intuitive Surgical has received an FDA green light for the fifth generation of its multiport da Vinci robot, following more than a decade of research and development.
Read the full article at: www.fiercebiotech.com
Continue Reading »Neuronetics’ magnetic stimulation cleared as first-line add-on therapy for adolescents with severe depression

The drug-free approach uses magnetic coils to generate pulses that aim to restart dormant synapse pathways in brain regions that help govern mood.
Read the full article at: www.fiercebiotech.com
Continue Reading »Ensuring Continued Availability of In-Vitro Diagnostics in UK, EU

The regulatory upheaval in the UK and EU, with the introduction of a new regulatory framework in the UK and the EU IVDR, represent both an opportunity and risk for bottlenecks.
Read the full article at: www.mddionline.com
Continue Reading »J&J adds Shockwave Medical to its cardiovascular collection with $13.1B deal
J&J MedTech sees Shockwave’s devices for cracking open calcified arteries as the ticket to its 13th billion-dollar platform.
Read the full article at: www.fiercebiotech.com
Continue Reading »Abbott’s TriClip Brings New Treatment Options to US Patients with Leaky Tricuspid Valves

Recent FDA approval of Abbott’s TriClip transcatheter edge-to-edge repair (TEER) device brings a new therapy option to U.S. patients suffering from tricuspid regurgitation (leaky tricuspid valve).
Read the full article at: www.mddionline.com
Continue Reading »After yearlong review, Titan Medical finds new home with Conavi Medical

The surgical robot developer Titan Medical has decided to combine itself with Conavi Medical, maker of imaging guidance tech for minimally invasive cardiovascular procedures.
Read the full article at: www.fiercebiotech.com
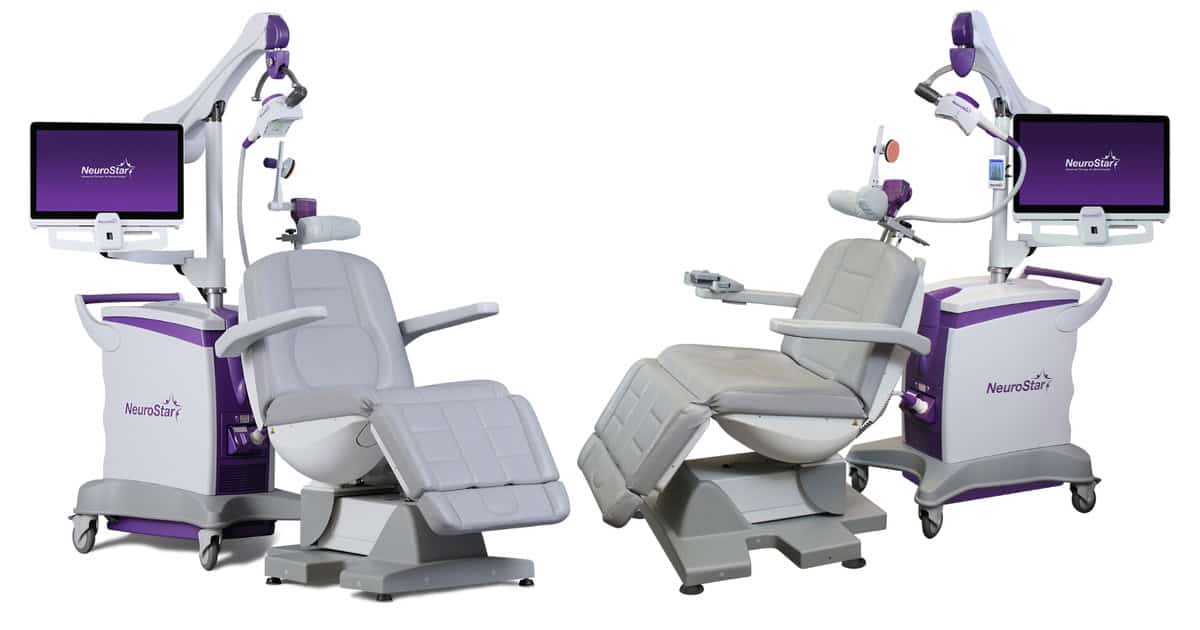
Continue Reading »Neuronetics Nabs FDA Clearance for NeuroStar Treatment in Adolescents
This is the company’s fourth FDA-cleared indication for the major depressive disorder treatment device.
Read the full article at: www.mddionline.com
Continue Reading »FDA clears Eko stethoscope AI to catch low ejection fraction
Developed in collaboration with the Mayo Clinic, the software can detect a key indicator of heart performance through Eko’s digitally enhanced stethoscopes.
Read the full article at: www.fiercebiotech.com
Continue Reading »