Edwards Lifesciences claims European approval for transcatheter tricuspid valve implant

The company described its Evoque valve as the first minimally invasive therapy of its kind to receive a regulatory green light.
Read the full article at: www.fiercebiotech.com
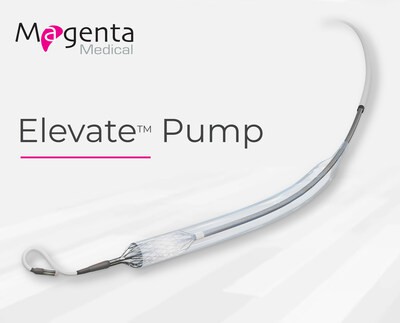
Continue Reading »Magenta Medical Reports Positive Results for US Early Feasibility Study of Elevate™ Heart Pump in Providing Temporary Mechanical Circulatory Support During High-Risk PCI Procedures
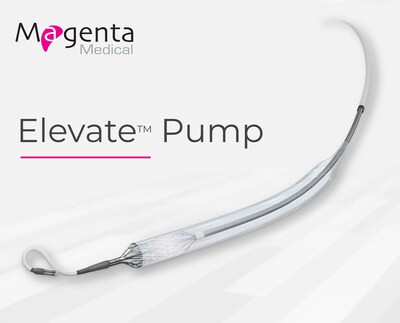
Magenta Medical Reports Positive Results for US Early Feasibility Study of Elevate™ Heart Pump in Providing Temporary Mechanical Circulatory Support During High-Risk PCI Procedures – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »Inari Bets Big on LimFlow

The $250 million acquisition (plus $165 million contingent on milestones) has been lauded as a “smart, timely buy of a pioneering, much-needed technology.”…
Read the full article at: www.mddionline.com
Continue Reading »Xēnix Medical Nabs 510(k) Clearance for neoWave Implants
The interbody fusion implants received FDA nanotechnology designation.
Read the full article at: www.mddionline.com
Continue Reading »FDA advisers wave through first CRISPR-based therapy

After Thursday’s advisory committee meeting, CRISPR and Vertex will now wait for an official FDA decision on exa-cel.
Read the full article at: www.fiercebiotech.com
Continue Reading »Attaining Device Usability Success Through Human Factors Validation

Technology is landing in the hands of more end users of various populations. Determining what defines useability is shifting as a result.
Read the full article at: www.mddionline.com
Continue Reading »SeaStar Nabs Breakthrough Device Designation for Hepatorenal Syndrome
The company’s Selective Cytopheretic Device may play a role in allowing kidneys to recover enough for patients to become liver transplant candidates.
Read the full article at: www.mddionline.com
Continue Reading »Insulet Omnipod 5 iPhone App FDA Cleared

The iPhone app offers users an expanded opportunity to control and manage the Omnipod and Dexcom G6 via a smartphone.
Read the full article at: www.mddionline.com
Continue Reading »iStar Brings Its Minimally Invasive Glaucoma Surgery device to Ireland
The minimally invasive glaucoma surgery (MIGS) marks an expansion of iStar’s commercial rollout in Europe.
Read the full article at: www.mddionline.com
Continue Reading »Medtronic claims FDA approval for defibrillator implant routed outside the heart and veins
Unlike other ICDs that are wired into beating hearts through the body’s veins, the Aurora’s electrical leads can be placed outside of the cardiac muscle and blood vessels to help reduce the risks of long-term complications.
Read the full article at: www.fiercebiotech.com
Continue Reading »