Design controls for medical devices are critical because they ensure safety, effectiveness, and regulatory compliance. These controls enable manufacturers to produce and innovate superior products that meet the needs of patients and healthcare providers.
At Omnica, we assist businesses of all sizes create top-quality medical devices. We understand how important it is for medical device manufacturers to comply with Food and Drug Administration (FDA) regulations and obtain ISO 13485 certification. Specifically, the Code of Federal Regulations Title 21 Part 820 Quality System Regulation (21 CFR 820 QSR) is mandatory for all medical device makers.
Therefore, to comply with regulatory requirements, our skilled team specializes in designing robust design control processes that include risk management, quality assurance, and traceability. We conduct comprehensive design reviews, verifications, and validations to meet the highest quality and safety standards.
What Are Design Controls?
Design controls are processes and activities for regulating medical device design and production. These safety measures ensure that the medical device is developed, manufactured, and tested in compliance with its intended use, user, and regulatory requirements. Design controls include several phases, documentation, and regulatory compliance — all of which contribute to producing safe, effective, and high-quality medical devices.
Do All Medical Products Need To Be Developed Under Design Controls?
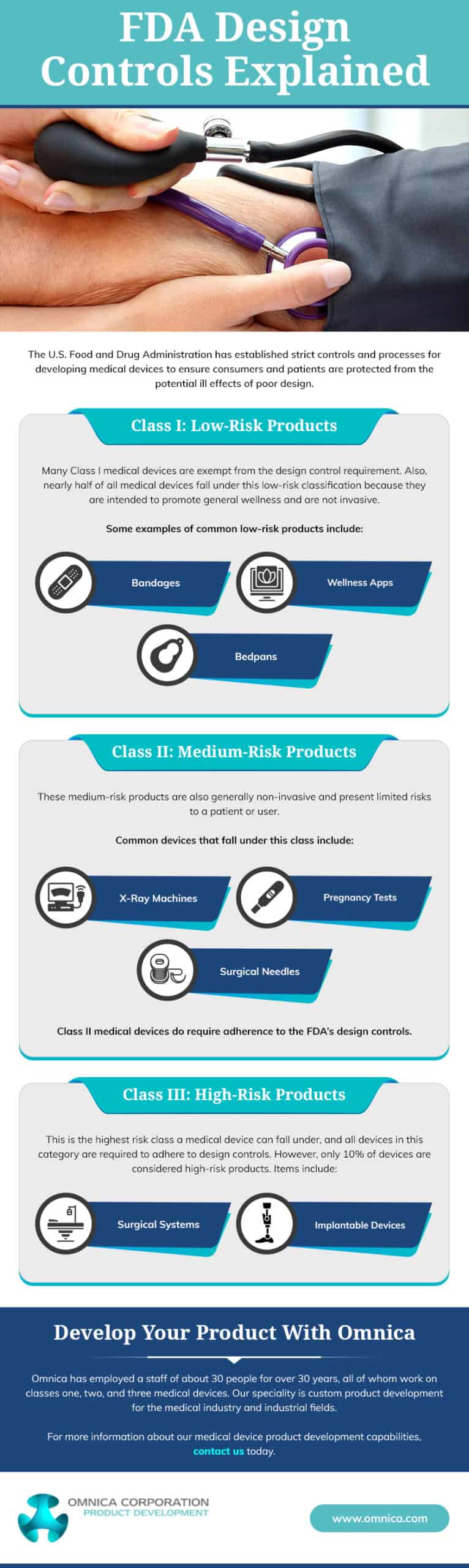
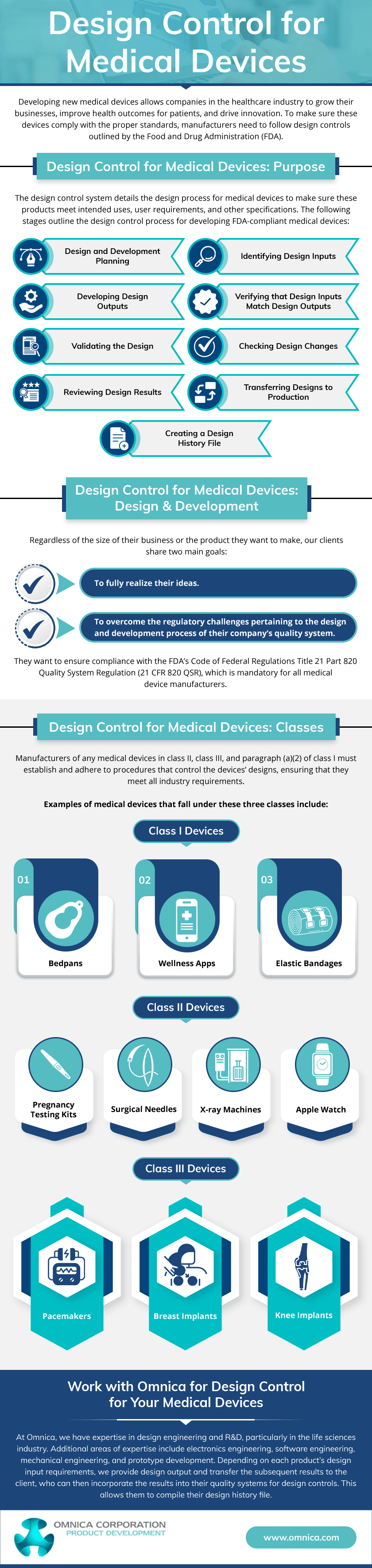
While design controls for medical devices are crucial, not all medical products must be produced under them. The regulatory requirements for design controls vary depending on the classification and risk level of the medical device. In the United States, the FDA categorizes medical devices into Class I, II, and III based on the risk level associated with the device.
Class III devices are considered high-risk and are subject to the most stringent regulatory requirements, including comprehensive design controls. On the other hand, Class II devices are subject to less strict design controls. Meanwhile, Class I devices may be exempt from design controls, though they are still required to meet general quality system requirements.
FDA and ISO Design Controls for Medical Devices
The design control rules provided by the FDA and ISO have similarities and differences. Here’s a more detailed description of the two regulations:
FDA Design Controls Regulation
In the United States, medical device development and manufacturing are governed by 21 CFR Part 820 of the FDA design controls rule. The design and development process emphasizes the significance of documentation, verification, and validation.
The FDA requires medical device manufacturers to establish and maintain procedures for each design control element. Its stages include:
- Design and development planning
- Design inputs
- Design outputs
- Design review
- Design verification
- Design validation
- Design transfer
Furthermore, the FDA requires manufacturers to document and keep records of all design-related activities, including changes to designs and design history files.
ISO Design Controls Regulation
The ISO 13485:2016 standard for medical devices emphasizes the importance of quality management and design controls. It highlights the need for robust documentation, traceability, and design reviews to ensure the safety and effectiveness of medical devices. Additionally, the standard emphasizes the importance of risk management, compliance with regulations, customer requirements, and usability considerations throughout the design and development process.
To demonstrate their dedication to quality and safety in the worldwide market, medical device manufacturers must comply with ISO 13485:2016. It establishes and maintains effective design and development processes to ensure that medical devices are safe, effective, and adhere to regulatory standards.
Phases Involved in Design Controls for Medical Devices
Design controls typically involve several phases to ensure the product is designed, manufactured, and tested following regulatory requirements and user needs. The main stages of design controls include the following:
Phase 1: Planning the Design
This phase involves establishing a plan for the design and development process, including defining objectives, identifying resources, and creating a timeline for the project.
Phase 2: Defining the Design Inputs
In this phase, user needs, intended use, and requirements for the medical device are identified and documented. This includes understanding the needs and expectations of the users, patients, and other stakeholders.
Phase 3: Creating the Design Outputs
This phase includes creating design specifications, drawings, and other documentation that define the design characteristics and features of the medical device.
Phase 4: Reviewing the Design
Design review is a critical phase where a cross-functional team reviews the design to ensure it meets the design input requirements and is safe and effective for its intended use. It helps identify any design issues, risks, or gaps that must be addressed before moving forward in the development process.
Phase 5: Verifying the Design
This phase involves conducting various tests and analyses to verify that the design output meets the design input requirements. It ensures the medical device is designed and manufactured correctly and performs as intended.
Phase 6: Validating the Design
Designs undergo validation to guarantee that medical equipment meets users’ needs and intended usage standards. This is typically done through real-world testing or clinical evaluations.
Phase 7: Transferring the Design
This phase involves transferring the design to production, including creating production specifications, transfer of design documentation, and training production personnel. This ensures the design is successfully transferred to manufacturing without losing quality or integrity.
Design Control Documentation and the Design History File (DHF)
Design control documentation is paramount in developing and regulating medical devices. It ensures that medical devices meet the standards set by regulatory bodies such as the FDA and ISO, ascertaining their approval and commercialization.
This documentation also provides regulatory bodies with an audit trail and ensures product quality and safety by tracing design inputs, outputs, and verification/validation processes. Thus, it helps manage risks by identifying, assessing, and mitigating medical device design and development potential hazards.
The Design History File (DHF) is a historical record of the design process and provides proof of conformity to regulations. It typically includes the following components:
Design and Development Plan
The design and development plan outlines the overall medical device development strategy. It includes the objectives, scope, and timeline of the project, as well as the roles and responsibilities of the team members.
Design Inputs
Design inputs are the specifications, requirements, and criteria defining the medical device’s intended use, performance, and safety. They serve as the foundation for the design and development process and provide a basis for design decisions.
Design Outputs
Design outputs are the results of the design and development process. They include drawings, specifications, models, prototypes, and other documents that represent the final design of the medical device.
Design Reviews
Design reviews are formal evaluations conducted at various stages of the design and development process to assess the progress and compliance of the medical device with the design inputs. They help identify and address any design issues or discrepancies.
Verification and Validation Records
Verification and validation records document the testing and evaluation activities performed to ensure the medical device meets the specified design inputs and requirements. This includes records of design verification, design validation, and any other relevant testing conducted.
Design Changes
Design changes refer to any modifications or revisions made to the design of the medical device during the development process. It records the reasons, justifications, and impacts of changes and the appropriate approvals obtained.
Risk Management Documentation
Risk management is a crucial aspect of medical device development. Therefore, per regulatory requirements, the DHF includes documentation related to risk management activities, such as risk assessments, risk mitigation strategies, and risk management plans.
Labeling and Packaging Documentation
The DHF may also include documentation related to labeling and packaging of the medical device, such as labeling specifications, packaging design, and labeling content. This is to ensure compliance with regulatory requirements and international standards.
Omnica Corporation: Your Reliable Partner for Medical Device Innovation
Ready to streamline your design control documentation and DHF process for your medical devices? Partner with Omnica, a leading design engineering and R&D firm with over 36 years of experience in the life sciences industry!
Contact us today to discuss your design project and see how our expertise can help you achieve regulatory compliance and innovative product design. Let us be your trusted partner in bringing safe and effective medical devices to market!