Medtronic Nabs CE Mark for Micra AV2 and Micra VR2 Leadless Pacemakers

The next generation pacemakers received FDA approval in 2023.
Read the full article at: www.mddionline.com
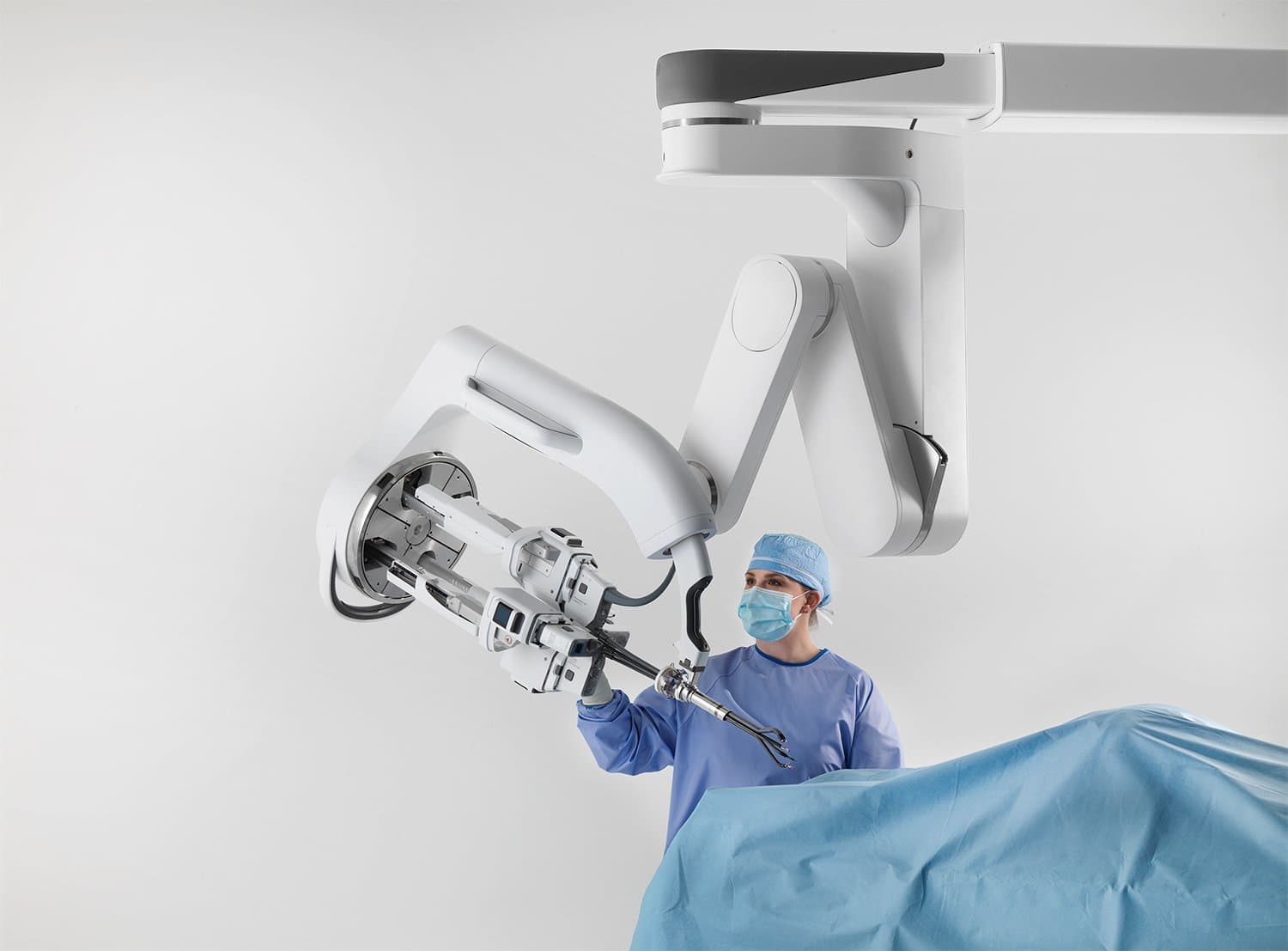
Continue Reading »Intuitive earns CE mark for single-port robot
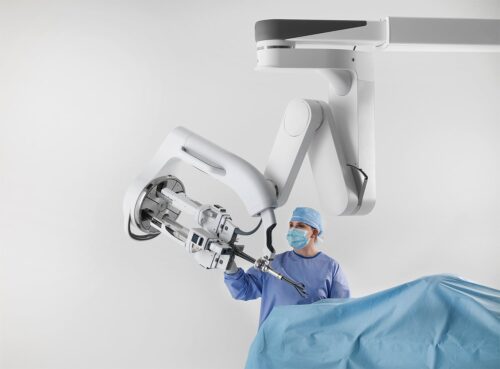
Da Vinci SP is cleared in the U.S. for prostate and ENT procedures, and it can now be used in Europe for an even wider range of surgeries.
Read the full article at: www.fiercebiotech.com
Continue Reading »Apple may nix pulse ox sensor from Apple Watch, Masimo says

The import ban affects only domestic sales of the Series 9 and Ultra 2 models of the Apple Watch, which Apple briefly stopped selling in December.
Read the full article at: www.fiercebiotech.com
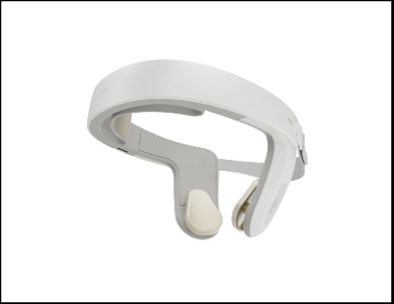
Continue Reading »Nexalin Technology Unveils Next-Generation HALO™ Clarity

Nexalin Technology Unveils Next-Generation HALO™ Clarity – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »Vaporized Hydrogen Peroxide Sterilization Gets FDA Established Category A Status

An Established Category A sterilization method has a long history of safe and effective use on medical devices, putting VHP on the same level as EtO and radiation.
Read the full article at: www.mddionline.com
Continue Reading »Abbott launches first human trials of its pulsed field ablation system for afib as the sector grows
Abbott announced it has begun conducting the first human procedures using its new Volt system, through a study of more than 30 patients in Australia.
Read the full article at: www.fiercebiotech.com
Continue Reading »FDA approves Medtronic’s rechargeable deep brain stimulator for Parkinson’s, epilepsy and more

Barely a month after Medtronic announced the European clearance of its rechargeable Percept RC neurostimulator, it has racked up a U.S. approval, too.
Read the full article at: www.fiercebiotech.com
Continue Reading »Nanowear collects FDA OK for wearable AI blood pressure monitor

The maker of a wearable garment for tracking heart rate, breathing and physical activity has obtained an FDA clearance for artificial intelligence-powered software that enables continuous monitoring of blood pressure without an inflatable cuff.
Read the full article at: www.fiercebiotech.com
Continue Reading »FDA grants first clearance to AI program for diagnosing idiopathic pulmonary fibrosis
The AI developer Imvaria has claimed a de novo clearance from the FDA for a digital diagnostic that analyzes chest CT scans for the signs of IPF.
Read the full article at: www.fiercebiotech.com
Continue Reading »Congressional Watchdog to Launch Probe into FDA Medical Device Recall Oversight

Two prominent senators called for an inquiry into the agency’s handling of device recalls through an open letter published last month.
Read the full article at: www.mddionline.com
Continue Reading »