Asensus Surgical seals the acquisition deal with Karl Storz

After previewing its agreement earlier this year, laparoscopic robotics developer Asensus Surgical has made its buyout official.
Read the full article at: www.fiercebiotech.com
Continue Reading »Providence Medical Technology, Inc. Announces FDA Clearance of CORUS™ Navigation Access System for Use with Medtronic’s StealthStation™ Surgical Navigation in Posterior Spinal Fusion

Providence Medical Technology, Inc.Announces FDA Clearance of CORUS™ Navigation Access System for Use with Medtronic’s StealthStation™ Surgical Navigation in Posterior Spinal Fusion – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »Women Underrepresented in Clinical Trials for High-Risk Medical Implants

A review published in JAMA Internal Medicine found that women have less than 50% — some down to 20% — median trial representation in common cardiovascular and orthopedic clinical trials.
Read the full article at: www.mddionline.com
Continue Reading »Recap of ‘s 2024 Ophthalmology Tech Forum: Innovation, Insight, and Cutting-Edge Content! •

The 2024 Ophthalmology Tech Forum, held at the VEA Newport, was a remarkable event that brought together industry leaders, top ophthalmologists, and key opinion leaders to discuss the future of eye care.
Read the full article at: octaneoc.org
Continue Reading »BD claims Edwards’ hospital monitoring portfolio for $4.2B

The deal includes Edwards’ Critical Care group and its 4,500 employees, which were previously being set up for a spinoff into an independent company.
Read the full article at: www.fiercebiotech.com
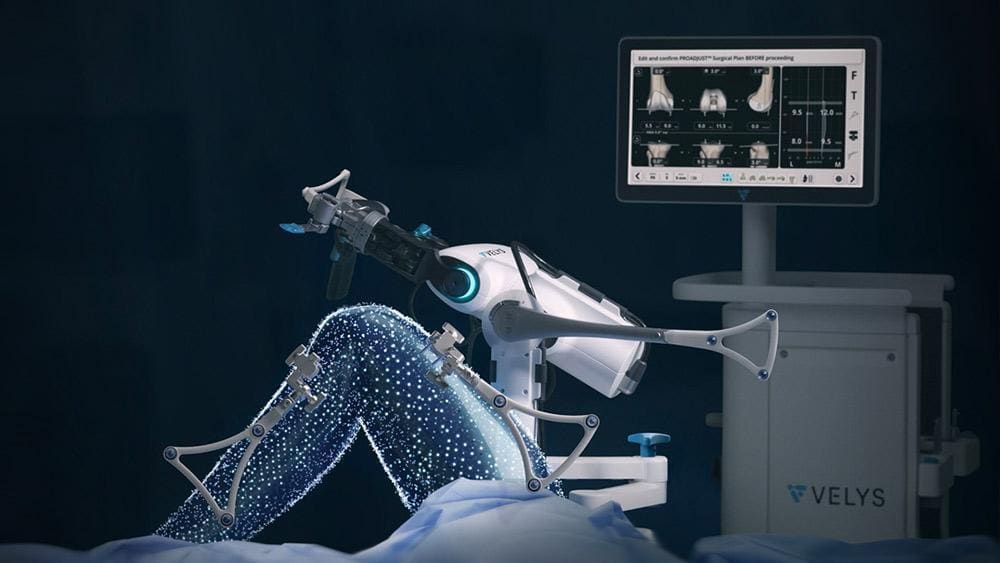
Continue Reading »J&J’s DePuy Synthes orthopedics robot cleared for partial knee replacements
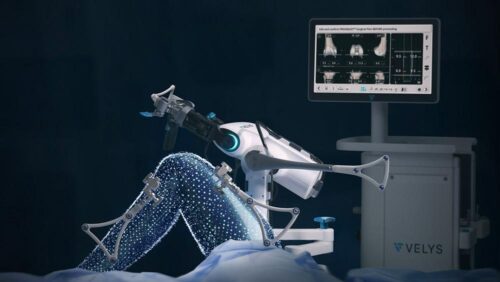
DePuy Synthes said partial replacements, also known as unicompartmental knee arthroplasty, have been underutilized due to the procedure’s complexity.
Read the full article at: www.fiercebiotech.com
Continue Reading »Microbot Robotic Surgical System FDA-Approved to Begin Human Trials

The Liberty system was designed for single use procedures to reduce exposure to radiation and eliminate user physical strain.
Read the full article at: www.mddionline.com
Continue Reading »Breathing New Life into Diagnostics: Plasmion’s SICRIT® Technology

Revolutionizing Non-Invasive Diagnostics with Plasmion’s SICRIT Breath Analysis.
Read the full article at: www.news-medical.net
Continue Reading »Study shows generative AI can speed up clinical trial enrollment for pennies per patient

Using a version of OpenAI’s GPT-4 program, the AI quickly combed through doctors’ notes and identified patients with heart failure who met study criteria.
Read the full article at: www.fiercebiotech.com
Continue Reading »Kardium nets $104M for its spherical pulsed field ablation catheter

After announcing positive clinical trial data from its pulsed field ablation treatment for irregular heart rhythms, Kardium has more news to share.
Read the full article at: www.fiercebiotech.com
Continue Reading »