Domus Diagnostics Wins $2.4M NIH RADx Tech Contract
The firm will use the funding to validate a rapid, low-cost, zero-power multiplex assay for COVID-19, flu A and B, and respiratory syncytial virus.
Read the full article at: www.360dx.com
Continue Reading »Super low-cost smartphone attachment brings blood pressure monitoring to your fingertips

Engineers have developed a simple 3D-printed attachment that clips over a smartphone’s camera and flash to measure blood pressure at the user’s fingertip. The clip works with a custom smartphone app and currently costs about 80 cents to make.
Read the full article at: www.sciencedaily.com
Continue Reading »What does ChatGPT mean for biology and the environment?

Exploring the potential of ChatGPT in the fields of biology and environmental science, this research paper investigates the implications and applications of using ChatGPT for advancing knowledge and understanding in these domains.
Read the full article at: www.news-medical.net
Continue Reading »Femtech Company Hits Another Growth Catalyst

Establishment Labs has received CE mark approval for minimally invasive surgical tools used in the company’s Mia Femtech procedure.
Read the full article at: www.mddionline.com
Continue Reading »Noah Hits Milestone to Strengthen Place in Surging Robotics Market

San Carlos, CA-based Noah Medical joins other companies that have made significant strides in surgical robotics in recent months.
Read the full article at: www.mddionline.com
Continue Reading »THINK Surgical’s TMINI Miniature Robotic System developed with Sagentia Innovation gains FDA 510(k) clearance

THINK Surgical’s TMINI Miniature Robotic System developed with Sagentia Innovation gains FDA 510(k) clearance – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »Corvia Medical Releases Two-year Clinical Trial Results Confirming Sustained Benefit and Safety of Its Atrial Shunt in Heart Failure Patients

Corvia Medical Releases Two-year Clinical Trial Results Confirming Sustained Benefit and Safety of Its Atrial Shunt in Heart Failure Patients – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »SunMed Finalizes Purchase of Vyaire Medical’s Respiratory Business
The complementary platforms will make SunMed a “one-stop source” for anesthesia and respiratory care consumables.
Read the full article at: www.plasticstoday.com
Continue Reading »Norlase Receives FDA 510(k) Clearance and CE Mark Approval For ECHO™ Green Pattern Laser
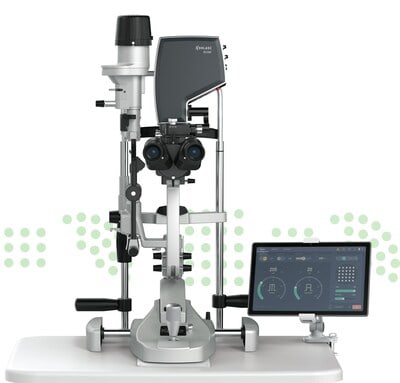
Norlase Receives FDA 510(k) Clearance and CE Mark Approval For ECHO™ Green Pattern Laser – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »Creating the Lab of the Future; What does it entail?

As part of our SLAS US 2023 coverage, we speak to Luigi Da Via, Team Leader in Analytical Development at GSK, about the lab of the future, and what it may look like.
Read the full article at: www.news-medical.net
Continue Reading »