Tinnitus treatment with the first neuromodulation device gets cleared by the FDA

When it comes to Neuromod Devices’ neurostimulation system to relieve tinnitus, the FDA is all ears.
Read the full article at: www.fiercebiotech.com
Continue Reading »FDA backs neurostim device for at-home depression treatment

According to a 2022 study, half of 410 participants saw their depressive symptoms reduced by at least 50% after using Sooma Medical’s device.
Read the full article at: www.fiercebiotech.com
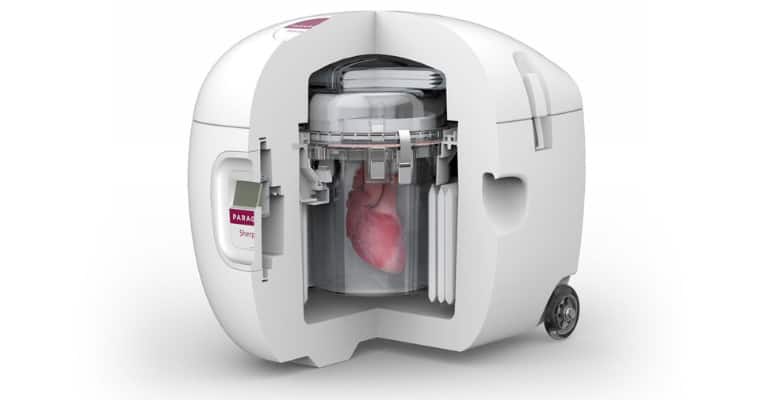
Continue Reading »Investors Are Taking Notice of Organ Preservation Company Paragonix
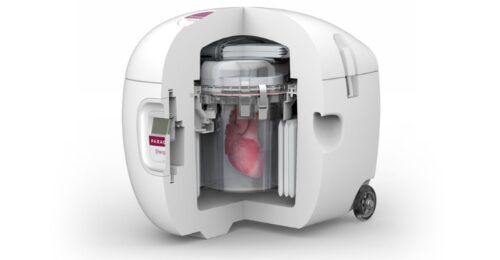
The company’s organ preservation methods represent a significant improvement over traditional ice and cooler practices still used today.
Read the full article at: www.mddionline.com
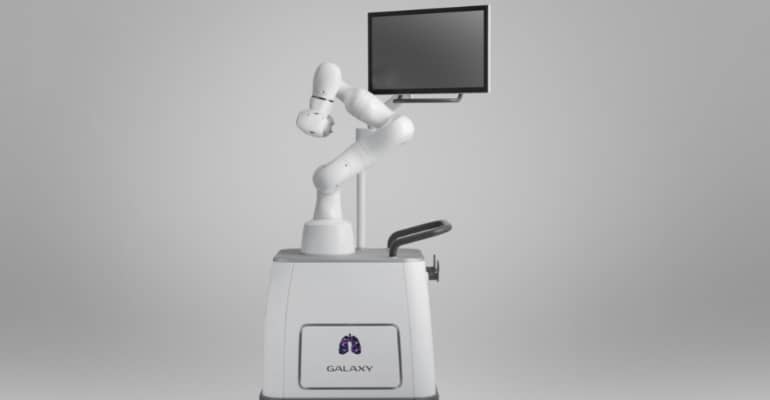
Continue Reading »Robotic Bronchoscopy Trial Launches in Australia
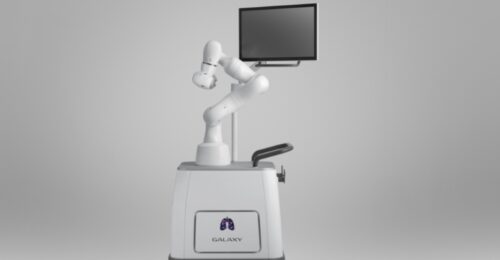
The first-in-human trial will evaluate Noah Medical’s Galaxy System, an integrated solution for navigated robotic bronchoscopy.
Read the full article at: www.mddionline.com
Continue Reading »Where is the Innovation in Sterilization?

Moving away from using ethylene oxide (EtO) in sterilization remains difficult with minimal market interest to address what is considered a niche problem.
Read the full article at: www.mddionline.com
Continue Reading »False Compliance with CISPR 11 Ends in $12 Million Company Fine

Take it from Advanced Bionics. If you’re going to use CISPR 11 to support findings in pre-market applications, it better be correct.
Read the full article at: www.mddionline.com
Continue Reading »The Bumpy Road to EU Medical Device Regulation (MDR)

The European Union’s Medical Device Regulation (MDR) transition has been extended to avoid critical medical device shortage.
Read the full article at: www.mddionline.com
Continue Reading »Roadblocks Still Exist in Transition to IVD Regulation

It is indisputable that the amending Regulation (EU) 2022/112, which extended the IVD Regulation’s transitional provisions, brought significant relief and protected patients, laboratories and healthcare systems from immediate shortages of needed IVD medical tests. Around 21% of today’s total IVD market is already certified under the IVDR. This represents a three-fold increase compared to July 2021.
Read the full article at: www.mddionline.com
Continue Reading »Are Supply Chain Pressures Easing for Medtech?

Here’s what GE Healthcare, Stryker, Abbott, Boston Scientific, and Intuitive Surgical expects in terms of the global supply chain in 2023.
Read the full article at: www.mddionline.com
Continue Reading »Infinity Neuro Receives CE Mark Approval for Their First Device to Treat Stroke
Infinity Neuro Receives CE Mark Approval for Their First Device to Treat Stroke – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »