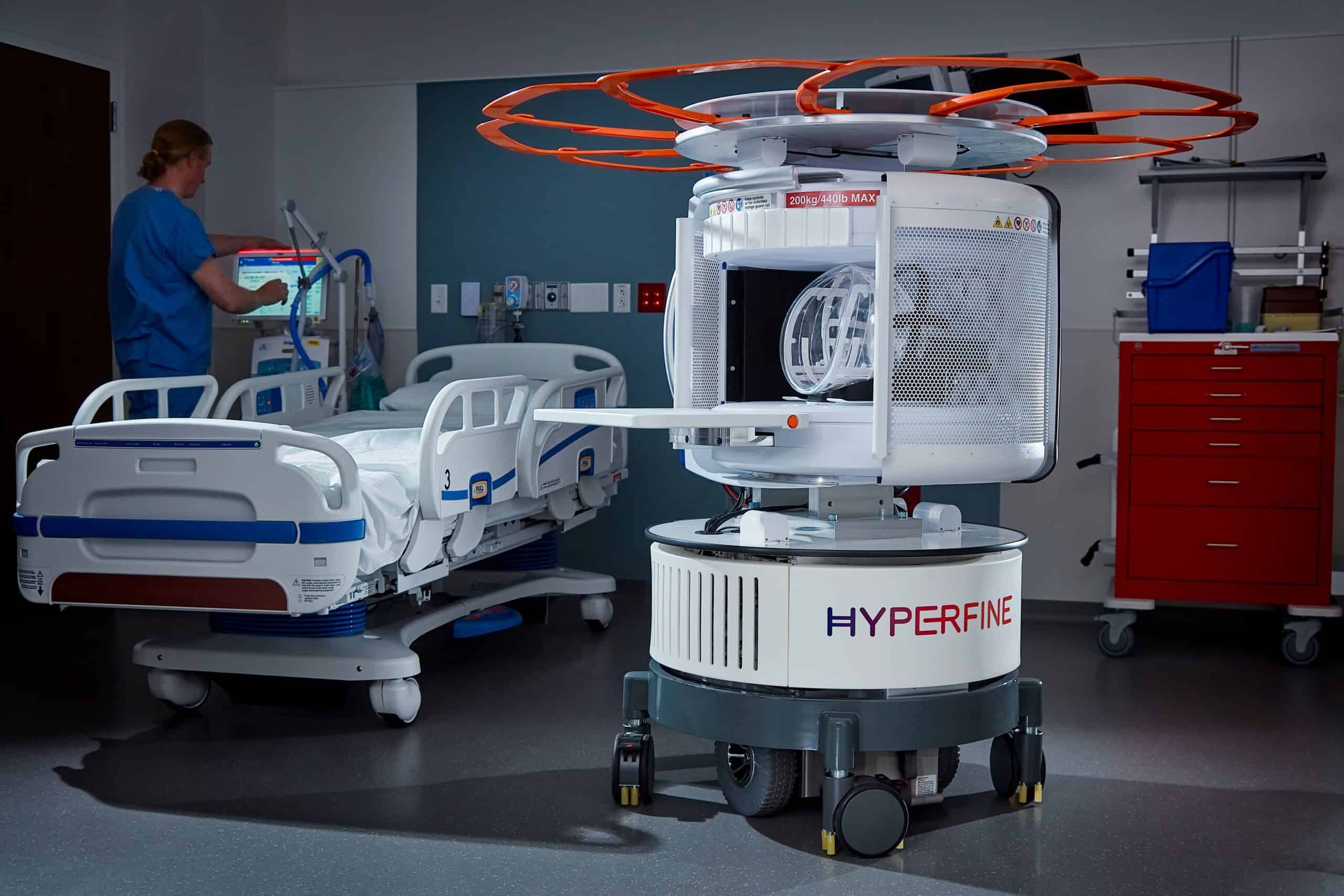
Hyperfine wins FDA nod to expand MRI image-sharpening AI
The latest nod marks Hyperfine’s eighth FDA clearance in the span of three years for its MRI scanner-on-wheels.
Read the full article at: www.fiercebiotech.com
Continue Reading »FDA to form advisory committee for digital health and AI

The FDA is enlisting a new panel of outside experts to help it get deeper into the AI weeds.
Read the full article at: www.fiercebiotech.com
Continue Reading »New tool reveals how drugs affect men, women differently — and will make for safer medications

Researchers have developed a powerful new tool to understand how medications affect men and women differently, and that will help lead to safer, more effective drugs in the future.
Read the full article at: www.sciencedaily.com
Continue Reading »MRI-Compatible Stereotactic Neurosurgery Robot

Dr. Gregory Fischer, in his keynote at BIOMEDevice Boston, discusses the journey from concept to commercialization for his MRI-compatible neurosurgery robot.
Read the full article at: www.mddionline.com
Continue Reading »The Approvals Keep Rolling in for Boston Scientific

The Marlborough, MA-based company has won approval for the latest generation of its Watchman LAAC device.
Read the full article at: www.mddionline.com
Continue Reading »Assessing the Cost & Regulatory Demands Cardiac Implantables

Mayo Clinic’s Marie Reyes discusses the…
Read the full article at: www.mddionline.com
Continue Reading »Apellis Announces U.S. FDA Approval of the EMPAVELI® Injector, a Device to Streamline Self-Administration

Apellis Announces U.S.FDA Approval of the EMPAVELI® Injector, a Device to Streamline Self-Administration – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »NIH, Industry Partner on Pediatric Medical Device Advancement

They will develop a national pediatric medical device ecosystem to narrow the gap in availability, options, and innovation seen in their adult counterparts.
Read the full article at: www.mddionline.com
Continue Reading »FDA Finalize Human Factors Guidance in Combination Medical Devices

The guidance focuses on applying HFE principles for medical devices combined with a drug or biological product.
Read the full article at: www.mddionline.com
Continue Reading »Field Medical nets $14M for ‘next gen’ pulsed field ablation

Just because pulsed field ablation therapy for afib has yet to make its mark in the U.S. doesn’t mean there’s reason to wait on developing version 2.0.
Read the full article at: www.fiercebiotech.com
Continue Reading »