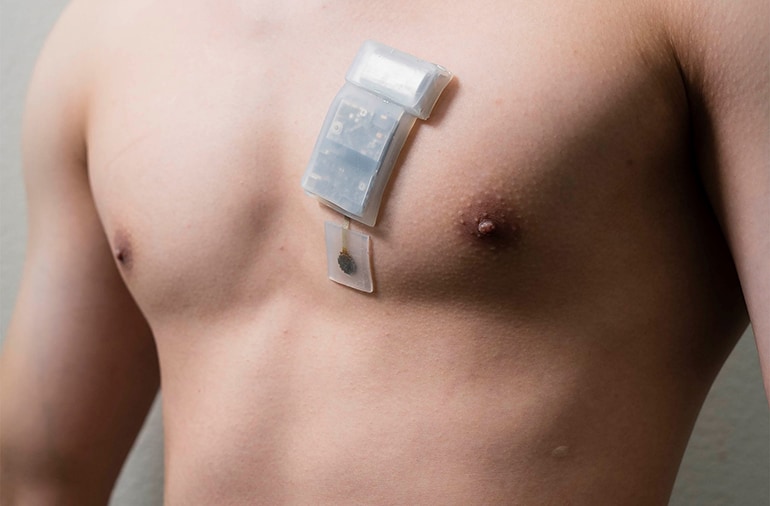
Wearable Ultrasound for Deep Tissue Monitoring
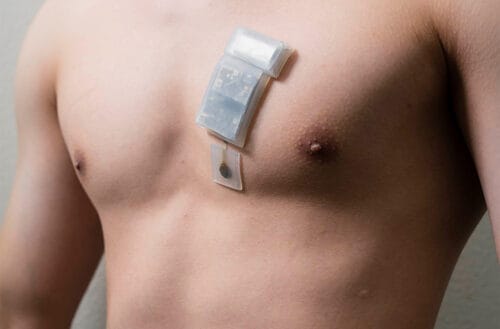
Researchers at the University of California San Diego have created a wearable ultrasound system that can monitor deep tissues, as far as 16.5 cm (6.5…
Read the full article at: www.medgadget.com
Continue Reading »Video Capsule Navigates the Stomach

Researchers at George Washington University have created a swallowable capsule containing a video camera that can assist in identifying lesions in the…
Read the full article at: www.medgadget.com
Continue Reading »Case builds for NeuroMetrix, Sudoscan devices in diabetic neuropathy
Handheld devices that can detect the telltale signs of peripheral neuropathy in people with diabetes could have another important function—predicting the risk that they will die within the next few…
Read the full article at: www.fiercebiotech.com
Continue Reading »Low-Cost Smartphone Finger Clip Measures Blood Pressure

Engineers at the University of California San Diego have developed a low-cost cuffless blood pressure monitor.The device is a clip that attaches over a…
Read the full article at: www.medgadget.com
Continue Reading »FDA Announces Additional Steps to Modernize Clinical Trials | FDA

The FDA is announcing the availability of a draft guidance with updated recommendations for good clinical practices aimed at modernizing the design and conduct of clinical trials.
Read the full article at: www.fda.gov
Continue Reading »Terasaki Institute Holds Grand Opening Celebration at New Research Center

Terasaki Institute Holds Grand Opening Celebration at New Research Center – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »Domus Diagnostics Wins $2.4M NIH RADx Tech Contract
The firm will use the funding to validate a rapid, low-cost, zero-power multiplex assay for COVID-19, flu A and B, and respiratory syncytial virus.
Read the full article at: www.360dx.com
Continue Reading »Super low-cost smartphone attachment brings blood pressure monitoring to your fingertips

Engineers have developed a simple 3D-printed attachment that clips over a smartphone’s camera and flash to measure blood pressure at the user’s fingertip. The clip works with a custom smartphone app and currently costs about 80 cents to make.
Read the full article at: www.sciencedaily.com
Continue Reading »What does ChatGPT mean for biology and the environment?

Exploring the potential of ChatGPT in the fields of biology and environmental science, this research paper investigates the implications and applications of using ChatGPT for advancing knowledge and understanding in these domains.
Read the full article at: www.news-medical.net
Continue Reading »Femtech Company Hits Another Growth Catalyst

Establishment Labs has received CE mark approval for minimally invasive surgical tools used in the company’s Mia Femtech procedure.
Read the full article at: www.mddionline.com
Continue Reading »