ReWalk to Demonstrate Stairs-Enabled Features at Abilities Expo
Larry Jasinski, CEO and managing director of ReWalk, discusses the introduction of the stair-enabled features to the US market.
Read the full article at: www.mddionline.com
Continue Reading »Omeq Medical bags FDA approval for epidural guidance device
A medical device that aims to make administration of epidurals more reliable and safer has been cleared by the FDA, giving its developer Omeq Medical its first approved product.
Read the full article at: www.fiercebiotech.com
Continue Reading »Smart Toilet Seat Wins FDA Nod to Monitor Heart Rate

Casana said it will also pursue additional filings including systolic and blood pressure.
Read the full article at: www.mddionline.com
Continue Reading »Top Mistakes in Medical Product Development

Underestimating the importance of human factors, funding, and classification can be the difference between product success and failure.
Read the full article at: www.mddionline.com
Continue Reading »Spotting Problems in 510(k) Submissions to Avoid Device Recalls

A study from the University of Minnesota offers guidance on how to avoid safety recalls for 510(k) devices.
Read the full article at: www.mddionline.com
Continue Reading »Great Britain to Delay New Medical Device Regulations

The UK government is now aiming to implement new medical device regulations for the Great Britain market in July 2025, pending Parliamentary approval.
Read the full article at: www.mddionline.com
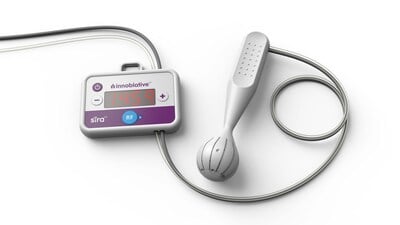
Continue Reading »Innoblative Receives U.S. FDA Breakthrough Device Designation for its SIRA RFA Electrosurgical Device
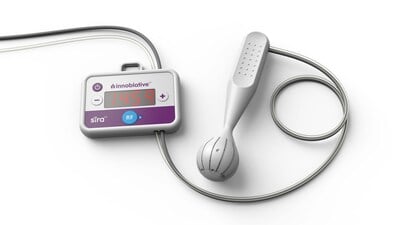
Innoblative Receives U.S.FDA Breakthrough Device Designation for its SIRA RFA Electrosurgical Device – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »What is the Minimum Log-Reduction Value for Medical Packaging?
A new study explores the minimum log-reduction value (LRV) for preventing microbial ingress for medical device sterile barrier systems.
Read the full article at: www.packagingdigest.com
Continue Reading »Warning Letter Serves as Reminder to Medical Device Industry

A London, England-based company received an FDA warning letter that should serve as an important reminder for all medical device manufacturers.
Read the full article at: www.mddionline.com
Continue Reading »The Difference Between Class 1, 2, and 3 Medical Devices
Click to Expand Medical devices are essential to healthcare, and their regulation is crucial to ensuring patient safety. They range from simple bandages and thermometers to complex diagnostic and therapeutic […]
Continue Reading »