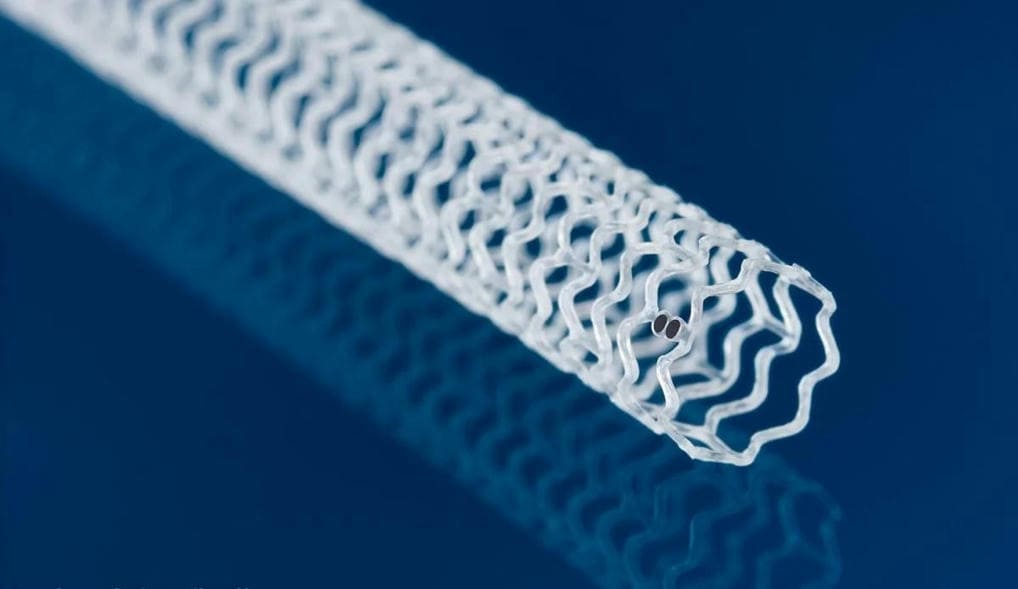
FDA approves Abbott’s dissolving stent for blocked arteries below the knee

Abbott has returned to the field of bio-absorbable, dissolving stents, and in somewhat uncharted territory. The Esprit BTK implant aims to treat the most severe form of peripheral artery disease, a condition known as chronic limb-threatening ischemia.
Read the full article at: www.fiercebiotech.com
Who We Are
Omnica Corporation is a privately-held design, engineering, and medical product development firm located in Irvine, California. The 28-person company is staffed with full-time employees and has been in operation since 1984. Our speciality is custom product development for the medical industry and industrial fields.
Our expertise is developing complex medical devices.Technical personnel at Omnica includes designers, mechanical engineers, electronic and software engineers, advanced R&D specialists, regulatory staff (for FDA documentation), machinists and model makers.