Scoop.it
Intuitive launches latest da Vinci robot, with force feedback controls
Posted on Leave a Comment

Intuitive Surgical has received an FDA green light for the fifth generation of its multiport da Vinci robot, following more than a decade of research and development.
Read the full article at: www.fiercebiotech.com
Continue Reading »Neuronetics’ magnetic stimulation cleared as first-line add-on therapy for adolescents with severe depression
Posted on Leave a Comment

The drug-free approach uses magnetic coils to generate pulses that aim to restart dormant synapse pathways in brain regions that help govern mood.
Read the full article at: www.fiercebiotech.com
Continue Reading »Ensuring Continued Availability of In-Vitro Diagnostics in UK, EU
Posted on Leave a Comment

The regulatory upheaval in the UK and EU, with the introduction of a new regulatory framework in the UK and the EU IVDR, represent both an opportunity and risk for bottlenecks.
Read the full article at: www.mddionline.com
Continue Reading »J&J adds Shockwave Medical to its cardiovascular collection with $13.1B deal
Posted on Leave a Comment
J&J MedTech sees Shockwave’s devices for cracking open calcified arteries as the ticket to its 13th billion-dollar platform.
Read the full article at: www.fiercebiotech.com
Continue Reading »Abbott’s TriClip Brings New Treatment Options to US Patients with Leaky Tricuspid Valves
Posted on Leave a Comment

Recent FDA approval of Abbott’s TriClip transcatheter edge-to-edge repair (TEER) device brings a new therapy option to U.S. patients suffering from tricuspid regurgitation (leaky tricuspid valve).
Read the full article at: www.mddionline.com
Continue Reading »After yearlong review, Titan Medical finds new home with Conavi Medical
Posted on Leave a Comment

The surgical robot developer Titan Medical has decided to combine itself with Conavi Medical, maker of imaging guidance tech for minimally invasive cardiovascular procedures.
Read the full article at: www.fiercebiotech.com
Continue Reading »Neuronetics Nabs FDA Clearance for NeuroStar Treatment in Adolescents
Posted on Leave a Comment
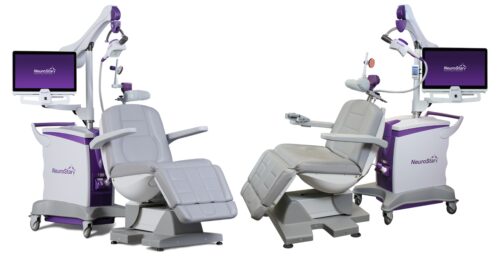
This is the company’s fourth FDA-cleared indication for the major depressive disorder treatment device.
Read the full article at: www.mddionline.com
Continue Reading »FDA clears Eko stethoscope AI to catch low ejection fraction
Posted on Leave a Comment
Developed in collaboration with the Mayo Clinic, the software can detect a key indicator of heart performance through Eko’s digitally enhanced stethoscopes.
Read the full article at: www.fiercebiotech.com
Continue Reading »20 Ways to Leverage AI in Medical Devices
Posted on Leave a Comment

Medical device engineers and artificial intelligence tech experts take to LinkedIn to tout the advantages of AI in medical devices and engineering.
Read the full article at: www.mddionline.com
Continue Reading »Abbott’s TriClip heart implant receives FDA approval for tricuspid valve repair
Posted on Leave a Comment

TriClip reinforces the valve separating the heart’s right atrium and ventricle by grasping together its flaps and forming a tighter seal.
Read the full article at: www.fiercebiotech.com
Continue Reading »