Project success in medical device product development depends on several intersecting aspects, including technological, legal, commercial, and medical. Every region and country has its own specifications of how medical devices are manufactured and what government agency oversees the process, such as the Food and Drug Administration (FDA) in the United States. At Omnica Corporation, our medical device product development process involves clearly defining any problems and mitigating potential risks to narrow down your design options, resulting in a safe and effective final product.
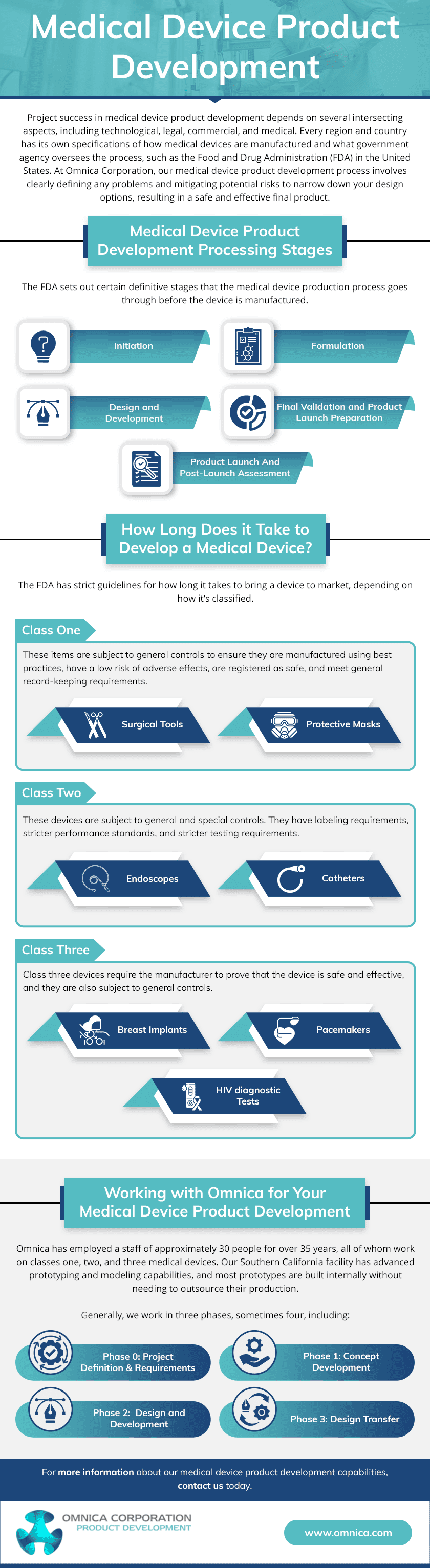
Medical Device Product Development Processing Stages
The FDA sets out certain definitive stages that the medical device production process goes through before the device is manufactured. These include:
- Initiation. This early part of the process involves looking at the device’s opportunity costs and risk analysis.
- Formulation. Next, the concept is made more concrete, and a feasibility study is conducted.
- Design and Development. Prototype design begins, as does a verification and validation process to ensure the design output matches the design input.
- Final Validation and Product Launch Preparation. Final validation tests are performed and the product is prepared for launch.
- Product Launch And Post-Launch Assessment. The product is launched and assessed for success.
How Long Does it Take to Develop a Medical Device?
How long it takes for a product to be developed primarily depends on its risk classification. The FDA has strict guidelines for how long it takes to bring a device to market, depending on how it’s classified.
- Class One. These devices pose little risk to users. Devices like this include surgical tools and protective masks. These items are subject to general controls to ensure they are manufactured using best practices, have a low risk of adverse effects, are registered as safe, and meet general record-keeping requirements.
- Class Two. Class two devices pose more of a risk to the public than class one and are subject to general and special controls. They have labeling requirements, stricter performance standards, and stricter testing requirements.
- Class Three. These are devices that sustain life or support it, are implanted in the body, or have a higher risk of danger to the user. For example, this class includes breast implants, pacemakers, and HIV diagnostic tests. Class three devices require the manufacturer to prove that the device is safe and effective, and they are also subject to general controls.
Medical Device Development Timeline
All medical devices start with researchers discovering a need. After that, the idea of the new device is drawn up, and a proof of concept document is created, which outlines how to prove the concept is feasible and workable. often, concepts are impractical, but ideas that show promise move to the later stages of product development.
Working with Omnica for Your Medical Device Product Development
Omnica has employed a staff of about 30 people for over 30 years, all of whom work on classes one, two, and three medical devices. Our Los Angeles facility has advanced prototyping and modeling capabilities, and most prototypes are built internally without needing to outsource their production. Not only does this decrease the manufacturing time for the device, but it also reduces the chance of mistakes. We are innovators, testers, and builders, and our process reflects that. Generally, we work in three phases, sometimes four, including:
- Phase 0. This phase is only needed if a customer doesn’t have clear documentation of what they intend to create. The result of Phase 0 includes specifying product requirements and details on what exactly we plan to develop.
- Phase 1. Concept Development. Once the design’s risks are identified, we remedy them and perform tests to ensure we have solutions for any risky aspects of the system. We then create a fully working alpha prototype, which we will test and collect data to plan the design of the final product.
- Phase 2. Design and Development. We build several beta prototypes, which undergo electrical prescreens, safety tests, and more. These prototypes are fully functional and look like the final product.
- Phase 3. Design Transfer. Once the product moves to high volume production, we’ll work with customers or their manufacturer to start the design transfer process.
For more information about our medical device product development capabilities, contact us today.