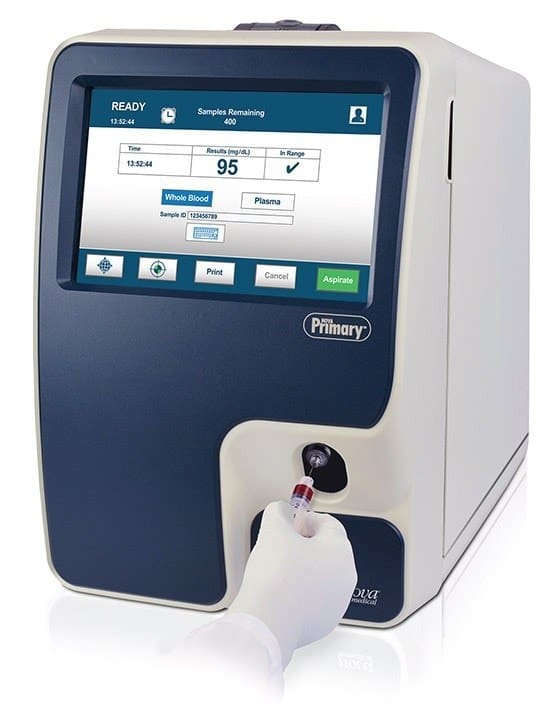
FDA Clears New Laboratory Blood Glucose Reference Analyzer from Nova Biomedical
The U.S. Food and Drug Administration (FDA) has cleared Nova Primary as a blood glucose reference analyzer.Nova Primary fills the need for a new glucose reference analyzer to replace the discontinued YSI STAT PLUS 2300 Glucose and L-Lactate analyzer…
Read the full article at: www.news-medical.net
Who We Are
Omnica Corporation is a privately-held design, engineering, and medical product development firm located in Irvine, California. The 28-person company is staffed with full-time employees and has been in operation since 1984. Our speciality is custom product development for the medical industry and industrial fields.
Our expertise is developing complex medical devices.Technical personnel at Omnica includes designers, mechanical engineers, electronic and software engineers, advanced R&D specialists, regulatory staff (for FDA documentation), machinists and model makers.