Great Britain to Delay New Medical Device Regulations

The UK government is now aiming to implement new medical device regulations for the Great Britain market in July 2025, pending Parliamentary approval.
Read the full article at: www.mddionline.com
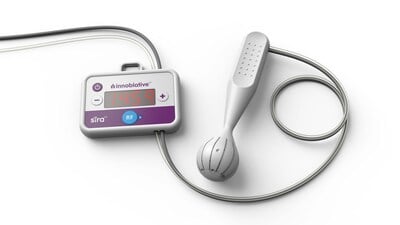
Continue Reading »Innoblative Receives U.S. FDA Breakthrough Device Designation for its SIRA RFA Electrosurgical Device
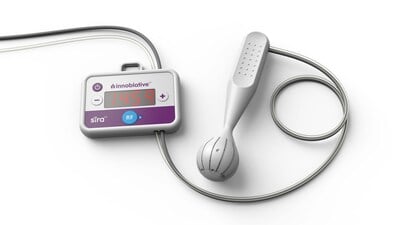
Innoblative Receives U.S.FDA Breakthrough Device Designation for its SIRA RFA Electrosurgical Device – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »What is the Minimum Log-Reduction Value for Medical Packaging?
A new study explores the minimum log-reduction value (LRV) for preventing microbial ingress for medical device sterile barrier systems.
Read the full article at: www.packagingdigest.com
Continue Reading »Warning Letter Serves as Reminder to Medical Device Industry

A London, England-based company received an FDA warning letter that should serve as an important reminder for all medical device manufacturers.
Read the full article at: www.mddionline.com
Continue Reading »The Difference Between Class 1, 2, and 3 Medical Devices
Click to Expand Medical devices are essential to healthcare, and their regulation is crucial to ensuring patient safety. They range from simple bandages and thermometers to complex diagnostic and therapeutic […]
Continue Reading »SeaStar Medical Announces Activation of First Clinical Site in Pivotal Trial with Selective Cytopheretic Device in Critically Ill Adults with Acute Kidney Injury

SeaStar Medical Announces Activation of First Clinical Site in Pivotal Trial with Selective Cytopheretic Device in Critically Ill Adults with Acute Kidney Injury – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
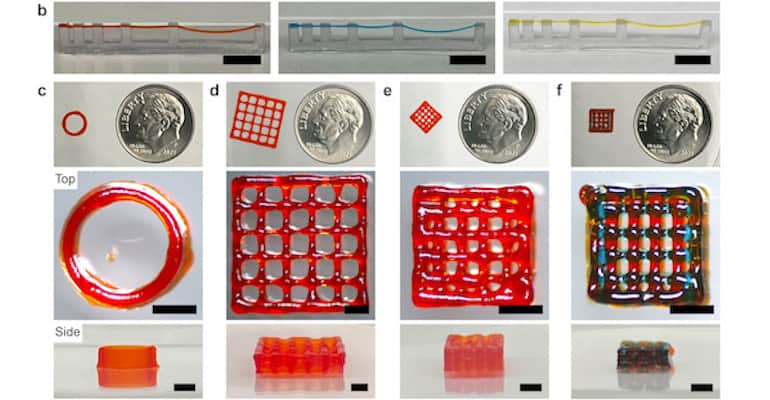
Continue Reading »Peptide-Based Inks for 3D Printing May Boost Regenerative Medicine
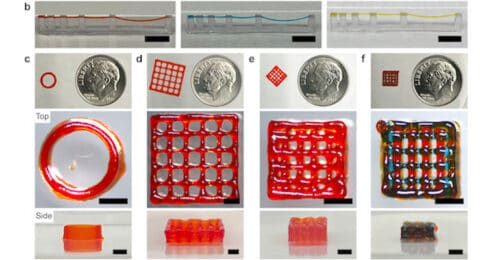
Hydrogel could be used for 3D printing structures that can grow active cells for transplant within living beings.
Read the full article at: www.designnews.com
Continue Reading »Noah Medical inundated with $150M in VC cash for its lung biopsy robot
Just weeks after securing an FDA clearance for its robotic lung surgery system, Noah Medical has been flooded with venture capital dollars, led by Softbank Vision Fund.
Read the full article at: www.fiercebiotech.com
Continue Reading »Get the Scoop on Your Child’s Poop via Microbiome Testing

This microbiome testing service turns poopy diapers into an interesting healthcare opportunity. TerraCycle Discovery offers the service.
Read the full article at: www.mddionline.com
Continue Reading »IceCure’s ProSense System Used in Endometriosis Cryoablation Study That Demonstrated Efficacy Rate of 92.8% to Avoid Secondary Surgery

IceCure’s ProSense System Used in Endometriosis Cryoablation Study That Demonstrated Efficacy Rate of 92.8% to Avoid Secondary Surgery – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »