How to Improve Instructions for Use – IFU Design

User-based research, enhanced visual elements, and early co-development of product and packaging support IFU designs for medical devices.
Read the full article at: www.packagingdigest.com
Continue Reading »FDA Clears ReddyPort® Non-Invasive Ventilation Device

FDA Clears ReddyPort® Non-Invasive Ventilation Device – read this article along with other careers information, tips and advice on BioSpace…
Read the full article at: www.biospace.com
Continue Reading »Fiber Probes to Investigate Brain-Gut Relationship
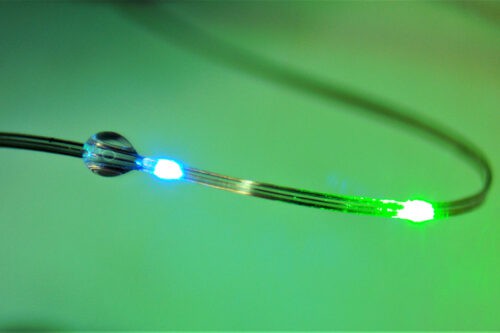
Engineers at MIT have developed a microelectronic probe that can measure and influence the behavior of neurons involved in the brain-gut axis.Neural…
Read the full article at: www.medgadget.com
Continue Reading »How to Build a Better PAP Machine
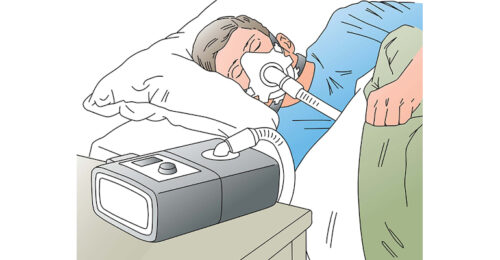
Sensor technology may help improve sleep apnea therapy adherence by making patients more comfortable with positive airway pressure (PAP) therapy devices.
Read the full article at: www.designnews.com
Continue Reading »French LVAD Maker Aims to Fill Void Left By Medtronic

Medtronic’s exit from the left ventricular assist device (LVAD) market left a huge void. A small French company wants to fill it.
Read the full article at: www.mddionline.com
Continue Reading »Microfluidic Chip Aids Tuberculosis Diagnosis

Researchers at the University of London have collaborated with QuantuMDx, a medtech company based in the UK, to develop a microfluidic diagnostic device…
Read the full article at: www.medgadget.com
Continue Reading »Beckman Coulter Life Sciences leading the way to help labs comply with confidence for upcoming annex 1 regulation deadline

Beckman Coulter Life Sciences, a global leader in laboratory automation and innovation, continues its relentless commitment to providing laboratories of all sizes with individualized and tailored support to meet the evolving list of regional and global compliance requirements.
Read the full article at: www.news-medical.net
Continue Reading »UK Health Officials Embrace AI Diagnostic Tools

With an investment of £21M, health officials launch an ambitious plan to leverage AI diagnostic tools for stroke, cancer, and heart conditions.
Read the full article at: www.mddionline.com
Continue Reading »Galen Robotics nets FDA de novo clearance for ENT surgical helper
The motorized ES system helps stabilize the surgeon’s direct manual control of their instrument, with the goal of reducing natural hand tremors.
Read the full article at: www.fiercebiotech.com
Continue Reading »BD earns updated FDA nod for beleaguered Alaris infusion pumps

Since early 2020, Alaris infusion pumps have received more than half-a-dozen hardware and software recalls. Now BD hopes to get them back on track.
Read the full article at: www.fiercebiotech.com
Continue Reading »